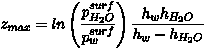You are on the first web site of Biotic Regulation. Since February 14, 2008 it is no longer updated. Welcome to www.bioticregulation.ru! |
Hot topic: Biotic pump of atmospheric moisture
|
Comments and responses |
Comment
De Melo Jorge Barbosa H. (2006) Interactive comment on
"Biotic pump of atmospheric moisture as driver of the hydrological cycle
on land" by A. M. Makarieva and V. G. Gorshkov. Hydrology and Earth
System Sciences Discussions, 3, S1418-S1424. www.cosis.net/copernicus/EGU/hessd/3/S1418/hessd-3-S1418.pdf
|
ResponseMakarieva A.M., Gorshkov V.G. (2006) Interactive comment on "Biotic pump of atmospheric moisture as driver of the hydrological cycle on land" by A. M. Makarieva and V. G. Gorshkov. Hydrology and Earth System Sciences Discussions, 3, S1449-S1458. www.cosis.net/copernicus/EGU/hessd/3/S1449/hessd-3-S1449.pdf In response to the comments of H. de Melo Jorge Barbosa: Note: the original order of comments of Dr. H. de Melo Jorge Barbosa was changed to match the order of our responses. Consult the corresponding PDF for the original text. | ||
|
As a physicist who have just recently changed field from astrophysics to meteorology I must say that I read this paper with great interest. It goes into the details of the physical meaning of meteorological concepts. However, I do have some concerns about some of the physical and meteorological arguments used by the authors which I would like discuss. For the time being, I will only discuss section 3.1, where most of the concepts are. I will enumerate my comments with the corresponding page and line number. P2638 l5 Quoting: "It equally acts on air volumes with positive and negative buoyancy,..." You started the section 3 saying that air meant dry air... so if there is no water vapor, there should be no such force, right? After that you say: "Quantitative consideration of this force, which creates upwelling air and water vapor fluxes (...) Why should this "force" act on dry air? If "dry air" molecules are in a state of hydrostatic balance they should not move... If only water vapor is out of equilibrium, only the H2O molecules should diffuse upwards trying to bring the vertical profile to equilibrium. And this would happen for all lapse rates between ~ 1.2km and 6 ~ 9.8 km which is quite a wide range (and therefore an important effect). In fact, you start section 3.2 citing Landau and Lifschitz about this subject. Could you give a more precise citation (chapter and page number) for equation 14? |
Dr. de Melo Jorge Barbosa raises a number of questions that we now address in the order of what we envisage as their physical importance, with more fundamental issues discussed first. 1) Evaporative force Comment to P3638, L5 (p. S1423) questions the existence of the evaporative force, which constitutes the basis of the physical mechanism of the biotic pump that we proposed. The comment says (quote): "If "dry air" molecules are in a state of hydrostatic balance, they should not move... If only water vapor is out of equilibrium, only the H2O molecules should diffuse upwards trying to bring the vertical profile to equilibrium." These statements contradict the bases of the kinetic theory of gases. Gas is a physical state where molecules are at large distances from one another as compared to the radius of molecular interaction. The state of gas is determined by the spatial distribution of molecules and by the velocity distribution of molecules that is formed in the course of molecular collisions. At low gas densities, as those in the terrestrial atmosphere, the ideal gas approximation is valid. Pressure of ideal gas does not depend on the properties of molecules (their mass, atomic structure, scattering length etc.) but is exclusively determined by temperature T and molar density N, p = NRT, that is, on temperature and the total number of gas molecules of whatever nature. Therefore, when molar density of a gas mixture deviates from equilibrium in a certain spatial area, local pressure of the gas mixture deviates from equilibrium as well. This leads to the appearance of a force acting on a unit volume of the gas mixture. This force is equal to the difference between the equilibrium pressure gradient and the existing (non-equilibrium) pressure gradient of the gas mixture. This process does not depend on whether the considered gaseous state is represented by only one gas or a mixture of several different gases. In the view of p = NRT, it does not matter whether molar density N deviates from equilibrium due to the change of molar densities of all mixture constituents or due to the change of the molar density of only one of them. Thus, when the molar density of water vapor deviates from equilibrium, this leads to the appearance of the evaporative force acting on the gas mixture as a whole, i.e. on the unit volume of moist air. | ||
|
|
The same effect can be illustrated by the following simple example. Let us take a closed jar divided into two compartments by a gas-impermeable partition and fill both compartments with atmospheric air. In the initial state molar densities and partial pressures of all gases are equal in both compartments; gases in the two compartments are in equilibrium. Let us now partially remove some gaseous component (e.g., oxygen) from the first compartment. This can be done, for example, by initiating a chemical reaction turning oxygen into some non-gaseous oxygen-containing chemical substances (e.g., solid oxides). Partial pressure of oxygen in the first compartment will drop. However, as far as the total pressure of gas mixture is equal to the sum of partial pressures of all its constituents, total air pressure in the first compartment will drop as well as compared to air pressure in the second, intact compartment. When we now remove the partition, acted upon by the force equal to the appeared pressure gradient, all gases from the second compartment (and not only oxygen!) will move to the first compartment to compensate for the oxygen and total pressure shortage there. Note that if we fill the first compartment in the jar with one gas, and the second compartment with a different gas, both gases having equal temperature and molar densities (and, hence, pressures), then, after removing the partition, a diffusion process will start mixing the gases and equating their molar densities in both compartments. There will be a diffusional flux of molecules of gas No. 1 to the second compartment, and of gas No. 2 -- to the first compartment. No pressure gradient, no force and no mass movements of gases will originate. In contrast, the process of equating non-equilibrium pressures of the gas mixture in the two compartments in the previous example is not a diffusion process, it is a dynamic mass movement of the gas mixture as a whole. On a related note, it is several times mentioned in the comments that the non-equilibrium distribution of water vapor induces "upward motion" of water vapor (see comment to P2636, L7, p. S1422), while in the considered comment to P3638, L5 it is said that the non-equilibrium distribution of water vapor will make water vapor "diffuse" to the upper atmosphere. We thus believe it is appropriate to emphasize the distinction between motion of gas as a whole (which may only occur under the action of some force) and the thermal chaotic motion of air molecules. The latter is the driver of molecular diffusion and occurs irrespective of whether a force is acting on the gas or not. In atmospheric physics the word "motion" (vertical motion, upward motion etc.) is conventionally applied to denote dynamic mass movements of air (i.e. motion of air as a whole). In this sense there cannot be any dynamic motions of water vapor in the otherwise motionless atmosphere, but only motions of moist air as a whole. To summarize, when the dry air constituents are in hydrostatic equilibrium, while water vapor is not, there is an upward-directed evaporative force acting on a unit volume of the entire gas mixture, moist air = dry air + water vapor. This force creates mass movement (or a dynamic (not diffusional) flux, as we referred to this movement in our response to Dr. Dovgaluk) of moist air in the atmosphere. As requested in the comment to P3638, L5, we now clarify how
expression (14) (p. 2638) for the evaporative force acting on moist air
is obtained from the Euler equation of the hydrodynamics. When there is a
force f acting on a unit gas volume with mass density r, this volume starts to accelerate in
accordance with Newton's law: r
dw/dt = f, where w is velocity.
The time derivative dw/dt describes the change of velocity
of the unit air volume, which moves in space. Hence, in the case of
vertical movement
We do not have the English text of Landau and Lifshitz (1987) at hand, but from our Russian edition we believe that equation (C1) can easily be located by its number, (2.4), in Section 2 "Euler equation" of Chapter 1 "Ideal liquid". Writing this equation for moist air (low index m),
pm = p + Concluding this issue, if there is no water vapor in the atmosphere, the evaporative force is zero. However, the evaporative force acting in one local area (e.g., over the ocean) can make the water-poor air in other areas (e.g., deserts) move as well, as prescribed by the continuity equation that governs atmospheric circulation. | ||
|
|
2) Saturation of surface air and atmospheric column | ||
|
P2634, L14 I don't think that immediately above the wet soil or open water the air is saturated of water vapor. If it was like that, no extra water would ever evaporate because the exchange of H2O molecules between the soil and this first saturated layer would always be in equilibrium. In the atmosphere, turbulence keeps recycling the surface air so that it is (almost) never totaly saturated. P2636, L7 The reasoning starting at line 7 is not clear enough.
For instance, how does a situation where
G >
Moreover, at line 11 you say that "excessive moister is
precipitating", but at line 8 you said that
pw(z) =
Another
question: why G >
Any given vertical profile of
humidity must be such that 0 < pvap(z)
≤psatH2O(z), for all z. When
G >
or
but the profile can be in equilibrium (but not necessarily) below this height. In any of these two cases, there will be an upward motion of water vapor trying to restore the equilibrium, within the part of the atmospheric column which is out of equi-librium. Notice that this upward motion does not depend on the profile being that of saturation. Supose, for instance, a particular profile p1 that starts at saturation at the surface:
This profile is not the saturation profile and since
0.5 *
Just
trying to summarize: you based your argument on the fact that the surface
is always saturated of water vapor and hence,
G >
The points that I (particularly) need some clarification in this argument are: 1) Is the surface always saturated? In line 20 of this page you say it is not. 2) Why the column should be fully saturated (and not like p1(z))? |
The condition G > To be brief, we start from the physically transparent formula (42.5) of Feynman et al. (1963), which gives the flux Fe of molecules (mol m-2 s-1) evaporating from unit of water surface area per unit time:
where v is molecular velocity (~ 500 m s-1 at room temperature for water molecules), 1/Va is the number of molecules per unit liquid volume (5.6´ 104 mol m-3 for water), and X is a dimensionless coefficient of the order of unity. When G < When G >
From (C3) it is clear that when w <<
we, then N1 = Ns,
i.e. concentration of water vapor at the surface is always saturated.
Given Ns ~ 0.7 mol m-3 at
15 oC and Fe ~ 0.3 mol m-2
s-1 as estimated from (C2) at
At the same time our estimate indicates that horizontal air fluxes with characteristic global wind speeds of the order of 10 m s-1 > we can substantially change relative humidity at the surface by mixing surface air from "drier" surfaces like poorly vegetated land with air from "wet" surfaces like oceans. The resulting mean global relative humidity at the surface, ~80% (Held and Soden, 2000), remains, however, fairly close to saturation, which reflects the spatial dominance of the hydrosphere on our planet. Distribution p1(z) suggested in the comment
to P2634, L14, p. S1422, is physically unrealistic. In this
distribution water vapor is compressed even further as compared to the
saturated case and has a scale height The non-equilibrium distribution of water vapor with Concluding our response to these comments: for questions regarding
the origin of lapse rate G >
Comment to 2636, L11, p. 1421 pw(z) =
| ||
|
|
3) Specific issues | ||
|
P2633, L20 Atmospheric air is not always in hydrostatic equilibrium (although it is always trying to be), and probably you don't think that it is. Better to have this paragraph rephrased. |
Comment to P2633, L20, p. S1419 | ||
|
P2634, L8 An isothermal atmosphere is not a good approximation, only the constant molar mass is... And only so because of the atmospheric circulation and turbulence which keeps the atmosphere mixed (Feynman), and there is no large sources or sinks of N2 or O2. |
Comment to P2634, L8, p. S1419 | ||
|
P2634, Eq. 9 - You should mention that in deriving eq (9) two
assumption are made: Vliq <<
Vgas (good) and
A more sophisticated expression is found in Bruining et al (2003):
where T is
temperature in K,
This represents a decrease of 1000J/mol for every 25 o C increase in temperature. If you believe this linear dependence is weak enough that you can neglect it, you should give the arguments for that.
and equation (10) follows immediately.
|
Comments to P2634, Eq. 9, p. S1419 and P2634, Eq. 10, p.
S1420 | ||
|
P2635, L14 I don't understand why do you need the approximation
exp(-z/H)
» 1
when deriving (11) from hw =
|
Comment to P2635, L14, p. S1420 | ||
|
P2635, last paragraph.
|
Comment to P2635, last paragraph, p. S1420 The general point that is overlooked in the
comment is that in the first case of
temperature dropping only slowly with height,
G <
| ||
|
P2636, L1
|
Comment to P2636, L1, p. S1421 | ||
|
P2637, L21 This assertion is not obvious at all and you should give a proof of that. In fact, consider a mesoscale convective complex. These systems which extend over large areas (typical size 100km x 100km) show strong updrafts associated with intense convection of buoyant parcels. Isn't this a counter example?
|
Comment to 2631, L21, p. S1422 Our statement that the Archimedes force cannot drive atmospheric motions over areas exceeding h2 is based on the physically transparent fact that h is the only relevant vertical length scale of the atmosphere. In our paper we show how the evaporative force can drive large-scale atmospheric circulation. We do not base our consideration on the Archimedes force and, hence, we do not need to prove this statement. This statement would need to be disproved in an attempt to show that the Archimedes force is able to drive large-scale atmospheric motions. The example of the observed extensive updraft regions in the real atmosphere is not a counter-example to our statement. We have shown (p. 2641, L3-19) that the evaporative force can lead to strong updrafts with vertical velocities of up to several dozens m s-1. | ||
|
REFERENCES
|
References Held, I.M. and Soden, B.J.: Water vapor feedback and global warming, Annu. Rev. Energy Environ. 25, 441-475, 2000. Feynman, R. P., Leighton, R. B. and Sands, M.: The Feynman lectures on physics, Vol. 1.4. Chapter 42, Section 1. Addison-Wesley Publishing Company, Reading, 1963. |
|